Muscle Spasticity
Controlling Muscle Spasticity due to Spinal Cord Injury
Spasticity is caused by damage or injury to the part of the central nervous system (the brain or spinal cord) that controls voluntary movement. This damage disrupts important signals between the nervous system and muscles, creating an imbalance that increases muscle activity or spasms.
Spasticity can make movement, posture, and balance difficult. It may affect your ability to move one or more of your limbs, or to move one side of your body. Sometimes spasticity is so severe that it gets in the way of daily activities, sleep patterns, and caregiving. In certain situations, this loss of control can be dangerous for the individual.
Intrathecal baclofen therapy (ITB) therapy is a treatment that may reduce some of the symptoms of your severe spasciticy.
Depending on your specific condition, a baclofen pump may impact your severe spasticity in different ways. Treatment may be even more effective when used in combination with physical, occupational, or speech therapy.
How a Baclofen Pump Reduces Severe Spasticity
Caused by Spinal Cord Injury or Spinal Cord Disease
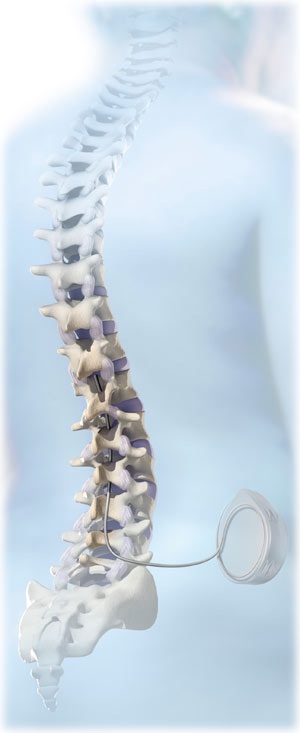
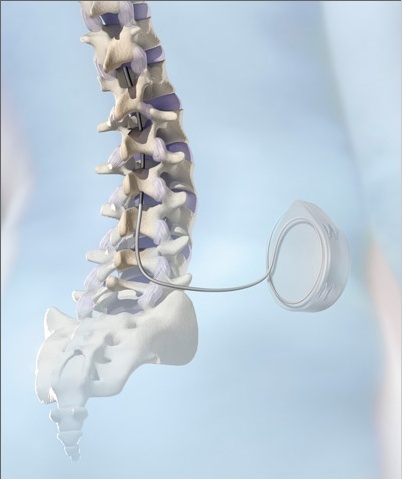
ITB therapy uses a surgically implanted programmable pump and catheter that delivers medication which helps relieve severe spasticity caused by spinal cord injury or spinal cord disease. This medication is a liquid form of baclofen that goes directly into the intrathecal space where fluid flows around the spinal cord.
Because baclofen is delivered directly to where it’s needed most in the spinal fluid, it relieves spasticity with smaller amounts of medication than when baclofen is taken orally. This method of delivery may help minimise side effects that can result from oral baclofen.
Therapy Components
The ITB therapy system consists of:
Pump – a surgically placed, battery-powered programmable pump with a reservoir infuses the drug at a programmed rate via the catheter
Catheter – a flexible silicone tube delivers medication from the pump to your body
Liquid baclofen – a drug that reduces severe spasticity for some people.
Treatment Options:
Intrathecal Medications
The US Food and Drug Administration approved the use of ITB TherapySM (Intrathecal Baclofen Therapy) for the treatment of spasticity of spinal and cerebral origin in 1992 and 1996, respectively. Since that time, it has been successfully used in the treatment of spasticity caused by stroke, cerebral palsy, multiple sclerosis, and acquired brain and spinal cord injuries. ITB Therapy is delivered directly into the thecal space with a three-part system made up of a surgically placed, programmable pump with a reservoir or storage area for the drug; a clear, flexible silicone tube or catheter; and a programming device. Because the drug is administered directly to its site of action—within the spinal cord—much less baclofen is needed than if the drug were taken by mouth. Because less of the medication is needed, the side effects of ITB Therapy, including drowsiness and sedation, are much milder than when baclofen is taken by mouth. ITB Therapy is usually combined with physical therapy and other forms of rehabilitation.
ITB Therapy is used to treat severe spasticity that is caused by damage to the brain or spinal cord. Candidates for ITB Therapy have severe spasticity that does not respond to conservative treatment with medications or have intolerable side effects at therapeutic doses. Pharmacotherapy should include, but need not be limited to, a trial of oral baclofen.
To determine whether ITB Therapy is expected to produce a helpful response, the patient undergoes an ITB Therapy screening test. The screening test for ITB Therapy requires the administration of a test dose of baclofen (typically with 50 mcg, usually not to exceed 100 mcg) via lumbar puncture into the thecal space. Peak effect of the drug usually occurs within four hours. Patients who respond positively to the test dose can be considered for ongoing ITB Therapy. During screening, patients must be monitored closely in a fully equipped and staffed setting, due to the risk of possible side effects.
Once it has been determined that ITB Therapy is likely to be effective in treating spasticity, a surgeon performs an operation to place a battery-powered pump. The SynchroMed® II pump, is smaller than the first style of pump that was used for ITB Therapy with a larger storage area (reservoir) that holds the baclofen. Two different pump sizes are available. The larger size allows for greater time between refills. The pump implantation requires two incisions: one in the lower abdomen to make a pocket for the pump under the skin, and another, smaller, incision in the lumbar region to insert the catheter. Exact placement of the pump differs with each patient, but it is generally implanted near the waistline, about one inch below the skin.
The surgeon inserts one end of the catheter into the intrathecal space using a spinal introducer. A myelogram is often used to confirm catheter placement. The catheter is then tunneled under the skin and the other end is connected to the pump. The pump is sewn into place under the skin in the pocket, and the incision is stitched closed. The tip of the catheter rests between the first and second lumbar vertebrae. The procedure typically lasts about 1 to 2 hours.
The entire hospital stay is usually 4 to 7 days, during which time the pump is programmed to deliver the best possible dose of baclofen to reduce muscle tone. There is some tenderness or soreness for several days after the operation, which can usually be controlled with pain medications. The implantable pump may cause a slight bulge in the abdominal wall, but many people report that they stop noticing this after several weeks. A notable decrease in the tone of spastic muscles is usually noticeable within several days of the operation, but significant improvements in function may take longer to be evident.
The dose of baclofen can be adjusted whenever necessary by reprogramming the pump in the doctor’s office. The pump also contains a programmable alarm that beeps softly when the reservoir is low or the batteries need replacing. The reservoir is refilled by injection as needed, usually every 1 to 6 months. When the batteries run low, about every 7 years, the entire pump is replaced.
The side effects of ITB Therapy are similar to those for baclofen given by mouth but, as mentioned previously, are typically milder because of the lower dose of medicine that is required with ITB Therapy. About 5% of people with ITB Therapy develop infections that require temporary removal of the pump. Other equipment-related risks include pump failure, catheter kinking or breakage, or movement of the catheter (dislodgement) so that the baclofen no longer reaches the intrathecal space. Mechanical defects or failure to refill the pump reservoir can lead to sudden interruption of baclofen treatment. In rare cases, this can cause a life-threatening withdrawal syndrome. To prevent this, families must be educated about the signs of baclofen withdrawal, and have a plan to respond to such possible emergencies.
Although it rarely happens, it is possible for the person receiving ITB Therapy to receive too much medication (overdose). A baclofen overdose may cause drowsiness, lightheadedness, slowed or difficulty breathing, seizures, loss of consciousness, and coma. In the event of an overdose, it is very important for the patient or caregiver to immediately contact the patient’s physician.
Activity Guidelines
More than 40,000 people worldwide have chosen ITB TherapySM (Intrathecal Baclofen Therapy) to manage their tight, stiff muscles associated with spinal cord injury, multiple sclerosis, stroke, cerebral palsy and brain injury.
While this therapy may help people be more independent, there are some guidelines to follow when living with ITB Therapy:
Metal detectors, theft detectors and security devices
Your pump may set off metal detectors at places like the airport, malls, schools, libraries, and other locations where metal detectors, theft detectors, or security devices are present. When approaching these devices, do not linger near or lean on the security screening device. Should you set off a metal detector/screening device, show your patient identification card to security personnel.
Hot baths and showers following surgery
Talk with your doctor to find out if you may shower immediately following surgery. You should not soak in a bath until after your stitches are removed and your incisions are healed. After the incisions are healed, a bath that is less than 39˚C (102˚F) will not affect your pump. The pressure in the pump reservoir is sensitive to the temperature. At higher temperatures, the pressure in the pump reservoir increases. If the increase is significant, the pressure can cause the pump to deliver too much medication. This may lead to a drug overdose.
Long airline flights and high-altitude activities, such as skiing or hiking in the mountains
Before engaging in activities at high altitudes (such as airline flights, skiing or hiking in the mountains), discuss the effects of low pressure with your doctor. Patients who live or travel at high altitudes are exposed to lower air pressures. With continued exposure to lower pressure, the flow rate of the pump may increase and then stay at the higher rate. If your doctor determines that such an increase in flow rate might pose an undue risk to you, your doctor can adjust your infusion prescription to offset this higher flow rate.
In rare cases, exposure to the lower pressures can cause the flow rate of the SynchroMed® II pump to exceed the programmed flow rate by more than 14.5% (15% for SynchroMed EL pumps) while the patient is exposed to the lower pressure. The infusion prescription in SynchroMed pumps can be changed for patients who will be exposed to lower pressures.
Traveling with a pump
Notify your clinician of any travel plans. Clinicians need this information to coordinate your care and pump refills, and prevent a loss of or change in therapy that could lead to serious injury or death.
SCUBA diving or hyperbaric chambers
Do not dive below 10 meters (33 feet) of water or enter hyperbaric chambers above 2.0 atmospheres absolute (ATA). Pressures below 10 meters (33 feet) of water (or above 2.0 ATA) could damage the pump, requiring surgery to replace the pump. To minimize damage to the pump when hyperbaric treatment is required, your doctor should fill the pump to capacity using the appropriate refill kit and maintain the current infusion prescription prior to exposure to hyperbaric conditions. Before diving or using a hyperbaric chamber, discuss the effects of high pressure with your doctor. As pressure increases, pump flow decreases. Continuing to increase the pressure will eventually lead to serious injury or death.
Hot tubs, steam rooms, saunas and tanning beds
If the temperature of a hot tub, steam room, sauna, or tanning bed is greater than 39˚C (102˚F), you should not use it. The flow rate of the pump will vary with body temperature. The flow rate increases as the temperature increases. If the increase is significant, the pump can deliver too much medication. This may lead to serious injury or death.
If you have additional questions, please don’t hesitate to ask your doctor or nurse; or you can contact Medtronic Patient Services.
IMPORTANT SAFETY INFORMATION ON ITB THERAPY:
Please follow your doctor’s instruction closely because a sudden stop of intrathecal baclofen therapy can result in serious illness (baclofen withdrawal symptoms) such as high fever, changed mental status, muscle rigidity, and in rare cases multiple organ-system failure and death. It is very important that your doctor be called right away if you experience any of the above symptoms.
It is important for you to keep your scheduled refill visits so you don’t run out of medication (baclofen) and to understand the early symptoms of baclofen withdrawal. Some patients are at more risk than others for baclofen withdrawal; consult with your doctor.
People who suffer from severe spasticity resulting from cerebral palsy, multiple sclerosis, stroke, traumatic brain injury, or spinal cord injury may be a candidate for ITB Therapy. If you have spasticity due to spinal cord injury or multiple sclerosis you must first fail oral baclofen. If you have experienced a traumatic brain injury you must first wait one year after the injury to be considered for ITB Therapy. A screening test will help show if you will respond to the intrathecal baclofen. You should not receive ITB Therapy if you have an infection, are allergic to baclofen, or your body size is too small to hold the implantable pump.
The implanted pump and catheter are surgically placed beneath the skin.
Surgical complications that you may experience include infection, meningitis, spinal fluid leak, paralysis, headache, swelling, bleeding, and bruising.
The most common and/or serious drug-related side effects of ITB Therapy include loose muscles, sleepiness, upset stomach, vomiting, headaches, and dizziness.
Pump failure may cause overdose or underdose of intrathecal baclofen.
The signs and symptoms of overdose include drowsiness, lightheadedness, respiratory depression (difficulty breathing), seizures, loss of consciousness, and coma.
Once the infusion system is implanted, device complications include catheter or pump moving within the body or eroding through the skin.
The catheter could leak, tear, kink, or become disconnected, resulting in underdose or no baclofen infusion.
Symptoms of underdose include increase or return in spasticity, itching, low blood pressure, lightheadedness, and tingling sensation.
These symptoms are often early indications of baclofen withdrawal. The pump could stop because the battery has run out or because of component failure.
The pump will sound an alarm when the pump needs to be filled with baclofen, replaced or if there is a problem with the pump.
Always inform any healthcare personnel that you have an implanted infusion system before any medical or diagnostic procedure such as MRI, diathermy, etc